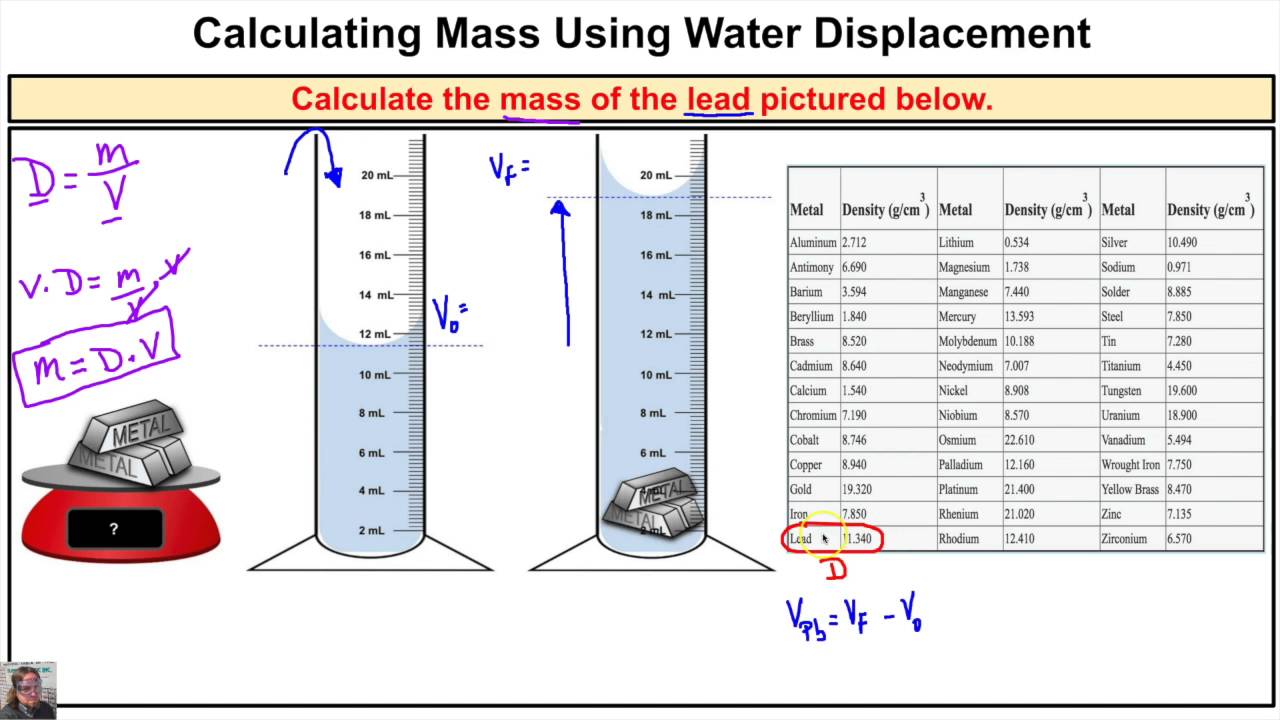
To measure the density of a sample of material, both the mass and volume of the sample must be determined. For both solids and liquids, a balance can be used to measure mass; however, methods for determining volume are different for solids and liquids. As liquids can flow and take the shapes of their containers, glassware such as a graduated cylinder or volumetric flask can be used to measure the volume of a liquid. The volume of an irregularly-shaped solid can be measured by submersion in a liquid — the difference in volume caused by addition of the solid is equal to the volume of the solid. Most solid substances are irregularly shaped, which complicates volume determination.
It is inaccurate, for example, to determine the volume of a powder by measuring its dimensions. A graduated cylinder containing a known volume of liquid is tared. The solid is added to the cylinder, and the total mass is weighed again to determine the mass of the solid.
Addition of the solid causes an upward displacement of the liquid, resulting in a new volume reading. The volume of the solid is equal to the change in volume due to liquid displacement (i.e., the difference in liquid volume before and after adding solid). One of the most common density measurements involves the determination of the geometric space occupied within the envelope of a solid material, including any voids, cracks, or pores.
This value only equals skeletal density when there are no internal openings in the material being measured. If the material has a uniform rectilinear shape, the volume it occupies can be calculated from measurements by caliper or ruler. However, a great majority of measurements involve complex shapes in formed materials such as ceramics, molded polymers, and granulated or pelleted products. In this case, the unknown volume may be determined by immersing it in a liquid and measuring the volume of liquid displaced, which is Archimedes principle .
To determine the density of an irregular solid in pellet form, add approximately 40 mL of water to a clean and dry 100-mL graduated cylinder. Place the cylinder on an analytical balance and tare. Add approximately 10 pellets, and record the new volume after the addition. The mass is only the pellets, as the rest have been tared. Make at least two additional sets of mass and volume measurements to calculate an average value of the density. The density for zinc was measured for three different samples.
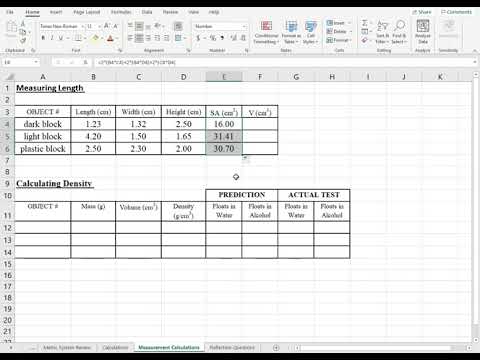
Note that, since the measurements were made in a graduated cylinder, which is less precise than a volumetric flask, the density has lower degree of precision. This demonstration illustrates the methods for measuring the density of solids and liquids. Using a volumetric flask and an analytical balance, the density of ethanol can be determined. Using a graduated cylinder, analytical balance, and water as the displaced liquid, the density of zinc metal can be determined. Table 1 lists results for the determination of the density of ethanol using a 50-mL volumetric flask.
Densities were calculated by dividing the measured mass by 50.0 mL. The mean measured density was 0.789 ± 0.001 g/mL. Table 2 lists results for the determination of the density of a sample of zinc metal using a 100-mL graduated cylinder and the liquid displacement method. Note that the measured densities are constant for both substances.
Table 2, in particular, demonstrates that density is independent of the amount of substance studied. This makes the purchase of the Density Kit accessory a very cost-effective investment. With the addition of a glass sinker of known volume, the Density Kit can also be used for determining the density of liquid samples. Of a substance is the ratio of the mass of a sample of the substance to its volume.
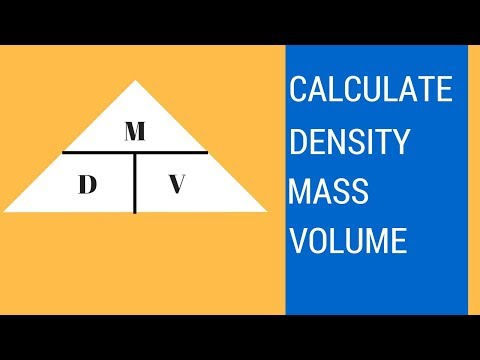
The SI unit for density is the kilogram per cubic meter (kg/m3). For many situations, however, this as an inconvenient unit, and we often use grams per cubic centimeter (g/cm3) for the densities of solids and liquids, and grams per liter (g/L) for gases. Although there are exceptions, most liquids and solids have densities that range from about 0.7 g/cm3 to 19 g/cm3 . Table \(\PageIndex\) shows the densities of some common substances. Relative density can be calculated directly by measuring the density of a sample and dividing it by the density of the reference substance. The density of the sample is simply its mass divided by its volume.
Although mass is easy to measure, the volume of an irregularly shaped sample can be more difficult to ascertain. One method is to put the sample in a water-filled graduated cylinder and read off how much water it displaces. Alternatively the container can be filled to the brim, the sample immersed, and the volume of overflow measured. The surface tension of the water may keep a significant amount of water from overflowing, which is especially problematic for small samples. For this reason it is desirable to use a water container with as small a mouth as possible. Relative density, or specific gravity, is the ratio of the density of a substance to the density of a given reference material.
Specific gravity for liquids is nearly always measured with respect to water at its densest (at 4 °C or 39.2 °F); for gases, the reference is air at room temperature (20 °C or 68 °F). The term "relative density" is often preferred in scientific usage. Volume is an amount of space, in three dimensions, that a sample of matter occupies. The number and the phase of the molecules in the sample primarily determine the volume of a substance. Volume will be measured in many ways in this course, but the units are usually milliliters or cubic centimeters .
Methods for determining or delivering precise volumes include volumetric pipets and pycnometers; less precise methods include burets, graduated cylinders, and graduated pipets. Volume is the amount of space an object occupies while density is the mass of an object per unit volume. You need to know the volume of an object before you can calculate its density. Calculating volume for regular objects can be done with a simple formula determined by the shape of the object. Common units for volume are cubic centimeters , cubic meters , cubic inches , and cubic feet . Once you have the volume, density is one more simple calculation away.
Common units for density are grams per cubic centimeter (g/cm3) or grams per milliliter (g/mL). The density of material shows the denseness of that material in a specific given area. A material's density is defined as its mass per unit volume. Density is essentially a measurement of how tightly matter is packed together.
It is a unique physical property for a particular object. The principle of density was discovered by the Greek scientist Archimedes. It is easy to calculate density if you know the formula and understand the related units The symbol ρ represents density or it can also be represented by the letter D. The density of solids can be measured by water displacement. The density of liquids can be measured by a device called a hydrometer.
My friend recently used one to ultimately find the alcohol content of beer he had brewed. I am on my phone so I can't provide links but there are a few processes and devices to measure density of substances without a scale and a ruler. Generally, the density of water (which is approximately about 1 gram/cubic centimeter) is taken as the standard value for calculating the density of substances. However, the SI unit of Density is measured using kilograms per cubic meter (kg/m3). A pycnometer is usually made of glass, with a close-fitting ground glass stopper with a capillary tube through it, so that air bubbles may escape from the apparatus.
This device enables a liquid's density to be measured accurately by reference to an appropriate working fluid, such as water or mercury, using an analytical balance. If the flask is weighed empty, full of water, and full of a liquid whose relative density is desired, the relative density of the liquid can easily be calculated. The particle density of a powder, to which the usual method of weighing cannot be applied, can also be determined with a pycnometer.
The powder is added to the pycnometer, which is then weighed, giving the weight of the powder sample. The pycnometer is then filled with a liquid of known density, in which the powder is completely insoluble. The weight of the displaced liquid can then be determined, and hence the relative density of the powder. To begin this procedure, place a clean and dry 50-mL volumetric flask on an analytical balance. After the measurement has stabilized, tare the balance.
Use a funnel to add approximately 45 mL of liquid to the flask. Use a Pasteur pipette to carefully add the final 5 mL of liquid, just until the bottom of the liquid's meniscus touches the line on the flask. Weigh the flask again and record the mass of the liquid.
Repeat the measurements at least twice to obtain additional values to calculate an average density. The average measured density was 0.789 g/mL, matching the literature value for ethanol. Because many objects are not regularly shaped their volume cannot be determined using this method. The volume of these irregularly shaped objects can be found by water displacement. A volume of water sufficient to cover the object is placed in a graduated cylinder and the volume read.
The object is added to the cylinder and the volume read again. The difference between the two readings is the volume of the object. This method is demonstrated using the same battery used above. Before you calculate density, calculate volume using the object's volume formula.
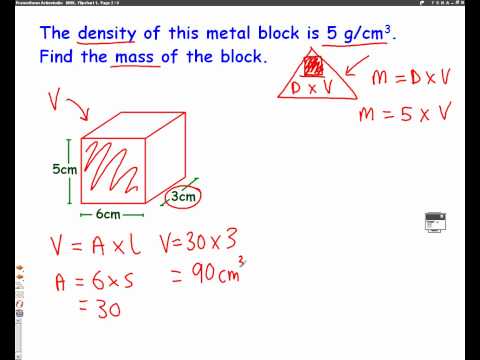
For example, for a rectangular prism, measure its length, width, and height, then solve for volume using the formula where volume equals length × width × height. After you have your object's volume, determine its mass by weighing it on a scale or with a balance. You can then calculate density by dividing the mass by the volume. Students use the water displacement method to find the volume of different rods that all have the same mass.
They calculate the density of each rod, and use the characteristic density of each material to identify all five rods. Then students consider the relationship between the mass, size, and arrangement of atoms to explain why different rods have different densities. Students will be briefly introduced to the periodic table.
Because the density of water in g/cm3 is 1.0, the SG of an object is will be almost the same as its density in g/cm3. However, specific gravity is a unitless number, and is the same in the metric system or any other measurement system. It is very useful when comparing the density of two objects. Since specific gravity is unitless, it doesn't matter whether the density was measured in g/cm3 or in some other units (like lbs/ft3).
Argon - Density and Specific Weight - Online calculator, figures and tables showing density and specific weight of argon, Ar, at varying temperature and pressure - Imperial and SI Units. To illustrate this issue we will examine hydrostatic method for determination of body density, which is often used for the purpose of estimating body fat percentage. Another practical method uses three measurements. Then a container filled to the brim with water is weighed, and weighed again with the sample immersed, after the displaced water has overflowed and been removed.
Subtracting the last reading from the sum of the first two readings gives the weight of the displaced water. The relative density result is the dry sample weight divided by that of the displaced water. This method allows the use of scales which cannot handle a suspended sample. A sample less dense than water can also be handled, but it has to be held down, and the error introduced by the fixing material must be considered. Calculate the uncertainty in the mass of water removed using error propagation.
Convert this mass to volume units by dividing by the density of water (use a precise value, specific to the water's temperature). This value equals the uncertainity in the volume of the metal cylinder. To measure the density of a sample of a substance, it is necessary to measure its mass and volume.
Mass is typically measured using an analytical balance, a precise instrument that relies on the force exerted by the sample due to gravity. The container to hold the sample is weighed and tared, so only the sample mass appears on the balance display when the sample is added to the container. Now that you have determined the shape, which formula to use, and made the necessary measurements, you can calculate volume. By plugging in the values of your measurements and doing the math.
Your finished product is the volume of your object.Remember to express your answer in cubic units. Whether you are using metric or SI, the unit of volume will always be cubic. Be sure to always add units to the end of your calculation.
The mass difference divided by the volume of the pycnometer is the density of the liquid.The sample is added to a U-shaped hollow glass tube in the device. The density of the sample is determined by measuring the frequency of vibration of the tube. The lower the frequency of vibration, the higher the density of the sample.
To perform pycnometry measurements, the mass of the cylinder and the mass of a flask filled with water to a mark (A, Fig. 3) are recorded. Water is displaced when the cylinder is inserted. The volume of water displaced is removed by pipet, thereby restoring the water level to the mark . The combined mass of the flask, remaining water, and cylinder is then measured.




















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.